Case Letter
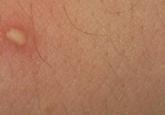
Identification of Cutaneous Warts: Cryotherapy-Induced Acetowhitelike Epithelium
Cutaneous warts are benign proliferations of the epidermis that occur secondary to human papillomavirus infection. The diagnosis of cutaneous...
Ted Rosen, MD; Anita Nelson, MD; Kevin Ault, MD
Dr. Rosen is from the Department of Dermatology, Baylor College of Medicine, Houston, Texas. Dr. Nelson is from the Department of Obstetrics and Gynecology, Harbor-UCLA Medical Center, Torrance, California. Dr. Ault is from the Department of Gynecology and Obstetrics, Emory University School of Medicine, Atlanta, Georgia.
The clinical studies reported in this article were sponsored and funded by Valeant Pharmaceuticals International, Inc. Dr. Rosen has served on the advisory board for and has received honoraria from Valeant Pharmaceuticals North America, LLC. Drs. Nelson and Ault have been advisors for Valeant Pharmaceuticals North America, LLC. Dr. Nelson also has received honoraria and is a consultant and speaker for Actavis and Bayer Health Care Pharmaceuticals; has received research grants from Agile Therapeutics and Bayer Health Care Pharmaceuticals; and is a consultant for Agile Therapeutics, ContraMed LLC, Merck & Co, Microchips Biotech, PharmaNest, and Teva Pharmaceutical Industries Ltd. She also is a speaker for Merck & Co; Pfizer, Inc; and Teva Pharmaceutical Industries Ltd.
Correspondence: Ted Rosen, MD, Department of Dermatology, Baylor College of Medicine, 1977 Butler Blvd, Houston, TX 77030 (vampireted@aol.com).

The median time to complete clearance was shorter in the 2 active treatment groups compared with placebo. For those participants who attained complete clearance, the median time to complete clearance ranged from 57 to 59 days in the imiquimod cream 3.75% groups (studies 1 and 2, respectively), 60 to 74 days in the imiquimod 2.5% cream groups (studies 1 and 2, respectively), and 76 to 81 days with placebo (studies 2 and 1, respectively).
Safety
Less than one-third of male participants in each treatment group experienced AEs during the studies. The incidence of serious adverse events (SAEs) and AEs leading to study discontinuation was low. In total, 4 participants (0.9% [3 in the imiquimod cream 2.5% group and 1 in the imiquimod cream 3.75% group]) had AEs that led to study discontinuation. Application-site reactions were reported in a total of 46 participants (10.3%). The incidence and severity of local skin reactions was mostly mild or moderate, similar in both active treatment groups, and higher than in the placebo group. Local skin reactions were coincident with the treatment period and rapidly decreased when treatment was concluded. There were no clinically meaningful trends in vital sign measurements or laboratory measurements.
Comment
Imiquimod cream 5% has been shown to be a safe and effective treatment of EGWs. Our study was designed to evaluate lower concentrations of imiquimod cream (2.5% and 3.75%), which may permit daily dosing and a shortened treatment course in men with EGWs.
Efficacy of imiquimod cream 2.5% and 3.75% was established through both primary and secondary end points, though only the higher concentration was significantly more effective than placebo in both studies. In addition, a number of participants who were not completely cleared following 8 weeks of treatment went on to be completely cleared at EOS, demonstrating continued activity of imiquimod despite cessation of active treatment.
Imiquimod cream 3.75% was particularly effective when compared to placebo, with 18.6% of participants completely cleared at EOS, though the PP (observed case) results (22.7%) may be more encouraging and can be used to motivate patients.
Although there are limitations in making direct comparisons between studies, complete clearance rates in our studies were lower than those reported previously with imiquimod cream 5%.17 Lower efficacy rates might be expected given the differences in methodology. In the 2 studies reported here, participants had to have no EGWs (baseline or new, treated or untreated) in any of the anogenital areas specified to be reported as having achieved complete clearance. In earlier studies with imiquimod cream 5%, not all anogenital regions were required to be treated, and any new EGWs arising during treatment were not included in the analysis.17 Also, our analysis focused purely on a male patient population in which efficacy results tend to be lower regardless of treatment modality employed.
Recurrence is another important issue in the treatment of EGWs. Although not studied specifically in a male population, recurrence rates of 16.7% to 17.7% were seen in the 3 months following successful treatment with imiquimod cream 2.5% and 3.75% in the 2 pivotal studies. These results were consistent with the recurrence rates reported following successful treatment with imiquimod cream 5%.17
In general, complete clearance rates increased in a dose-dependent manner. Complete clearance rates were lower in the male subpopulation across all treatment groups compared to those previously reported in females,24 which was consistent with prior results reported for imiquimod cream 5% as well as other topical treatments.17 It has been suggested that this difference may be due in part to the distribution of female EGWs in areas of less keratinization. Complete clearance rates in the current analysis tended to be higher in male participants with baseline EGWs in anatomic sites with less keratinized skin such as the perianal, perineal, or glans penis areas.
Daily application of imiquimod cream 2.5% and 3.75% generally was well tolerated. Most reported AEs were mild or moderate, and few participants discontinued because of AEs. Few SAEs were reported and none were considered to be treatment related. There was no difference in the incidence rates of AEs between the 2 active treatments. The incidence of SAEs and study discontinuations was much lower than previously reported in the female cohort of these 2 studies.24
Conclusion
In conclusion, 2 well-controlled studies of males with EGWs who were treated for up to 8 weeks with imiquimod cream 2.5% and 3.75% applied daily demonstrated good tolerability and superior efficacy to placebo in complete clearance of all baseline and newly arising warts in addition to reducing EGW counts.

Cutaneous warts are benign proliferations of the epidermis that occur secondary to human papillomavirus infection. The diagnosis of cutaneous...

Lasers have become an important part of the dermatologist’s arsenal for the treatment of skin diseases. As such, familiarity with the usage and...
Vestibular papillomatosis (VP) is a benign condition of the female genitalia that may be mistaken for condyloma acuminatum (genital warts).
