Case Letter
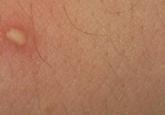
Identification of Cutaneous Warts: Cryotherapy-Induced Acetowhitelike Epithelium
Cutaneous warts are benign proliferations of the epidermis that occur secondary to human papillomavirus infection. The diagnosis of cutaneous...
Ted Rosen, MD; Anita Nelson, MD; Kevin Ault, MD
Dr. Rosen is from the Department of Dermatology, Baylor College of Medicine, Houston, Texas. Dr. Nelson is from the Department of Obstetrics and Gynecology, Harbor-UCLA Medical Center, Torrance, California. Dr. Ault is from the Department of Gynecology and Obstetrics, Emory University School of Medicine, Atlanta, Georgia.
The clinical studies reported in this article were sponsored and funded by Valeant Pharmaceuticals International, Inc. Dr. Rosen has served on the advisory board for and has received honoraria from Valeant Pharmaceuticals North America, LLC. Drs. Nelson and Ault have been advisors for Valeant Pharmaceuticals North America, LLC. Dr. Nelson also has received honoraria and is a consultant and speaker for Actavis and Bayer Health Care Pharmaceuticals; has received research grants from Agile Therapeutics and Bayer Health Care Pharmaceuticals; and is a consultant for Agile Therapeutics, ContraMed LLC, Merck & Co, Microchips Biotech, PharmaNest, and Teva Pharmaceutical Industries Ltd. She also is a speaker for Merck & Co; Pfizer, Inc; and Teva Pharmaceutical Industries Ltd.
Correspondence: Ted Rosen, MD, Department of Dermatology, Baylor College of Medicine, 1977 Butler Blvd, Houston, TX 77030 (vampireted@aol.com).

Secondary end points were 75% or more and 50% or more reduction in EGW count, change in EGW count from baseline, and 12-week sustained clearance rate.
Safety
Safety assessments of AEs, both volunteered and elicited, were made throughout the study.
Study Oversight
The study was conducted in accordance with the ethical principles specified in the Declaration of Helsinki and in compliance with the requirements of local regulatory committees. All participants provided written informed consent.
Statistical Analysis
Statistical analysis for intention-to-treat (ITT) imputations was made for missing data points using last observation carried forward (LOCF). Complete clearance rates and partial clearance rates were analyzed using Cochran-Mantel-Haenszel statistics stratified by center and by gender for the overall population analyses. The percentage change in EGW count was analyzed using analysis of covariance. All statistical analyses were performed using SAS software (version 9.1.3).
Results
Study Population
Study characteristics by treatment group are summarized in the Table. Overall, 447 male participants (225 from study 1 and 222 from study 2) were included in the study. The majority of participants (84.1%) had EGWs on the penile shaft with only 1 affected region in just over half of participants (51.9%). Most participants (70.2%) were 35 years or younger, and approximately half had a baseline EGW count of 7 or less (50.6%). More than 20% of participants had an affected wart surface area greater than 150 mm2 at baseline, and in more than 60% of participants, the duration from first diagnosis of EGWs to enrollment in the study was more than 1 year.
Primary Efficacy End Point
Imiquimod cream 3.75% was statistically superior to placebo in study 1 and study 2 (P=.015 and P=.019, respectively)(Figure) in providing complete clearance of all EGWs (baseline or new) at EOS. Imiquimod cream 2.5% was only statistically superior to placebo in study 2 (P=.034). Importantly, there were a number of participants who did not achieve complete clearance at EOT who continued to improve posttreatment. The percentage of participants treated with imiquimod cream 3.75% and 2.5% who were completely cleared at EOT was 12.0% (14.7% study 1 and 9.1% study 2) and 7.1% (7.2% study 1 and 7.1% study 2), respectively, compared to complete clearance rates of 18.6% (20.0% study 1 and 17.0% study 2) and 14.3% (13.3% study 1 and 15.3% study 2), respectively, at EOS (ITT population).
Complete clearance rates (defined as the proportion of participants by the end-of-study [EOS] visit with zero external genital warts in all anogenital anatomic areas) in the intention-to-treat (ITT) population (last observation carried forward [LOCF])(A) and per-protocol (PP) population (OC [observed case])(B), including both individual and pooled study data. |
In both studies complete clearance rates were significantly higher (P<.019 both studies) with imiquimod cream 3.75% compared with placebo at weeks 10 through 16 (EOS). In study 2, complete clearance rates were significantly higher (P<.049) with imiquimod cream 2.5% compared to placebo from week 14 to week 16 (EOS). Complete clearance rates were highest in participants treated with imiquimod cream 3.75% who had EGWs in the perianal region or on the glans penis (28.6% and 33.3%, respectively); however, the number of participants in both groups was relatively small.
Overall, 18.8% of participants took rest periods. Complete clearance rates were higher in men who took a rest period (26.5% and 27.3% for imiquimod cream 3.75% and 2.5%, respectively), perhaps reflecting a more brisk immunological response. The frequency, duration, and number of dosing days prior to the rest period were similar in the active treatment groups and lower in the placebo group.
There was a tendency for older participants (ie, >35 years) and those with lower baseline EGW counts (ie, ≤7) to respond better. Participants treated with imiquimod cream 3.75% also tended to respond best to treatment when only 1 anatomic area was affected.
Secondary Efficacy End Points
The proportion of male participants with at least a 75% reduction in EGW count from baseline at EOS was statistically superior with imiquimod 3.75% compared to placebo in study 1 and study 2 (P=.001 and P=.008, respectively). Statistical superiority also was apparent with imiquimod cream 2.5% versus placebo in study 2 (P=.013). Overall, 20.2% (18.1% study 1 and 22.4% study 2) and 27.3% (30.5% study 1 and 23.9% study 2) of participants achieved at least a 75% reduction in wart count at EOS with imiquimod cream 2.5% and 3.75%, respectively (pooled data).
Percentage change in EGW count from baseline at EOS was 35.8% and 24.1% with imiquimod cream 3.75% in study 1 and study 2, respectively, both significantly better than placebo (P=.002 and P=.011, respectively). Change in EGW count following treatment with imiquimod cream 2.5% was only significant in study 2 (P=.001).

Cutaneous warts are benign proliferations of the epidermis that occur secondary to human papillomavirus infection. The diagnosis of cutaneous...

Lasers have become an important part of the dermatologist’s arsenal for the treatment of skin diseases. As such, familiarity with the usage and...
Vestibular papillomatosis (VP) is a benign condition of the female genitalia that may be mistaken for condyloma acuminatum (genital warts).
