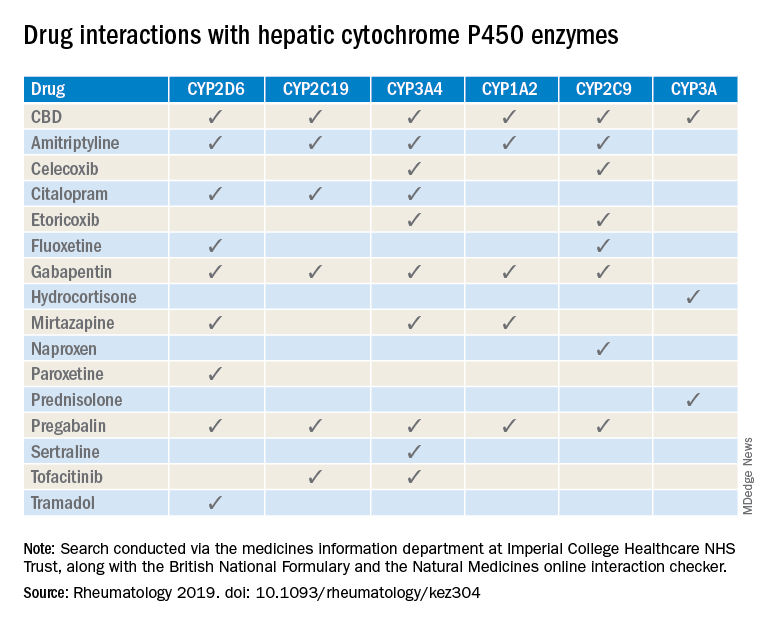
A number of medications commonly prescribed by rheumatologists may interact with cannabidiol oil, investigators at the Imperial College Healthcare NHS Trust, London, reported.
“Patients are increasingly requesting information concerning the safety of CBD oil,” Taryn Youngstein, MD, and associates said in letter to the editor in Rheumatology, but current guidelines on the use of medical cannabis do “not address the potential interactions between CBD oil and medicines frequently used in the rheumatology clinic.”
The most important potential CBD interaction, they suggested, may be with corticosteroids. Hydrocortisone and prednisolone both inhibit the cytochrome P450 enzyme CYP3A, but CBD is a potent inhibitor of CYP3A, so “concomitant use may decrease glucocorticoid clearance and increase risk of systemic [corticosteroid] side effects,” the investigators wrote.
CBD also is known to inhibit the cytochrome P450 isozymes CYP2C9, CYP2D6, CYP2C19, CYP3A4, and CYP1A2, which, alone or in combination, are involved in the metabolization of naproxen, tramadol, amitriptyline, and tofacitinib (Xeljanz), according to a literature search done via the college’s medicine information department that also used the British National Formulary and the Natural Medicines online interaction checker.
The Janus kinase inhibitor tofacitinib is included among the possible interactions, but the other Food and Drug Administration–approved JAK inhibitor, baricitinib (Olumiant), is primarily metabolized by the kidneys and should not have significant interaction with CBD, Dr. Youngstein and associates said. Most of the conventional synthetic and biologic disease-modifying antirheumatic drugs, including methotrexate, hydroxychloroquine, adalimumab (Humira), and abatacept (Orencia), also are expected to be relatively free from CBD interactions.
This first published report on interactions between CBD oil and common rheumatology medications “highlights the importance of taking comprehensive drug histories, by asking directly about drugs considered alternative medicines and food supplements,” they said.
The investigators declared no conflicts of interest, and there was no specific funding for the study.
SOURCE: Wilson-Morkeh H et al. Rheumatology. 2019 July 29. doi: 10.1093/rheumatology/kez304.