Study details
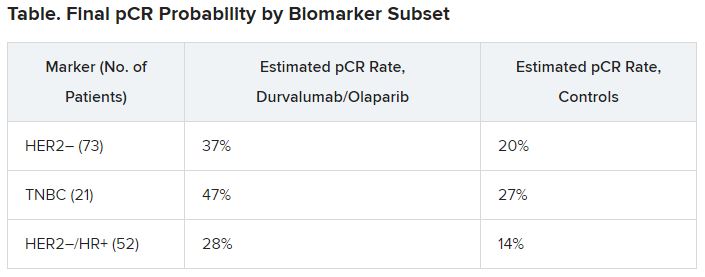
The I-SPY-2 results that Pusztai reported at the AACR meeting were based on an analysis of 73 HER2-negative patients, including 21 patients with TNBC and 52 with HR-positive tumors with Mammaprint high-risk features. These patients underwent treatment with durvalumab 1500 mg every 4 weeks for three cycles, olaparib 100 mg twice daily for weeks 1 through 11, and paclitaxel 80 mg/m2 weekly for 12 weeks, followed by AC chemotherapy (doxorubicin and cyclophosphamide) for four cycles.
The 299 patients in the control arm received paclitaxel and chemotherapy only.
In all three biomarker subsets studied, durvalumab and olaparib increased pCR rates compared with controls, as shown in the table.
The probability that the combination was superior to control in each subgroup approached 100%, Pusztai noted.
Adverse events with the combination were consistent with known side effects of the drugs, he commented. Immune-related grade 3 adverse events occurred in 19% of patients in the combination therapy arm, compared with 1.6% in the control arm.
Higher pCR rates were seen in the subset of immune-rich tumors among all cancer subtypes and in both study arms.
“Exploratory analysis suggests several potential predictive markers of durvalumab/olaparib benefit over chemotherapy alone,” Pusztai reported.
These markers included Mammaprint MP2 (ultra high) versus MP1 in HR+/HER2– tumors, and low CD3/CD8 gene signature ratio, high macrophage/Tc-class 2 gene signature ratio, and high proliferation signature, all of which were associated with higher pCR rates in the experimental arm among patients with TNBC.
The trial was supported by the William K. Bowes Jr Foundation, Foundation for the NIH, Give Breast Cancer the Boot, UCSF, the Biomarkers Consortium, IQVIA, the Breast Cancer Research Foundation, Safeway, California Breast Cancer Research Program, Breast Cancer Research–Atwater Trust, and Stand Up to Cancer. Pusztai has received honoraria and consulting fees from AstraZeneca and other companies. Munster has received research and travel support from and has served on the scientific advisory boards of AstraZeneca and other companies.
This article first appeared on Medscape.com.