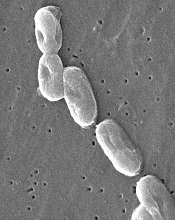
Image courtesy of
CDC/Janice Carr
The US Food and Drug Administration (FDA) has announced a Class I, nationwide recall of Nurse Assist Inc.’s normal saline flush syringes due to incidents of Burkholderia cepacia contamination.
Nurse Assist reported that patients developed B cepacia bloodstream infections while receiving intravenous care using the company’s prepackaged saline flush syringes, so the company voluntarily recalled all unexpired lots of the product.
This recall was first announced last October, but, yesterday, the FDA classified it as a Class I recall and said use of these syringes may cause serious injuries or death.
Nurse Assist said it is investigating the link between the syringes and the infections with the FDA, the US Centers for Disease Control, and various state health departments.
Until the investigation can be completed, all healthcare facilities with affected product should discontinue use and return the product to the supplier.
The normal saline flush is a plastic syringe filled with 0.9% sodium chloride (a 12 mL IV flush syringe with a 3 mL, 5 mL, or 10 mL fill volume). It is used to clear out medical devices that deliver medicine directly into the veins of a patient through a needle or catheter.
All unexpired lots of this product are covered by the recall. Product Numbers 1203, 1205, and 1210 are packaged 30 syringes to an inner carton and 6 inner cartons in a case (180 syringes).
For product number 1210-BP, 100 syringes are packaged in an inner carton with 4 inner cartons in a case (400 syringes). Lot code information can be found on the outer case panel, the back panel of the inner carton, and on each syringe label.
The lots being recalled were distributed to customers and distributors between February 16, 2016, and September 30, 2016. Product can be identified by the labeling on the packaging and device.
Customers with questions can contact Nurse Assist Inc. at 1-800-649-6800 ext. 10, Monday through Friday, between the hours of 8 am and 5 pm, Central Time, or via email at ProductRemovalInfo@nurseassist.com.
Healthcare professionals and consumers can report adverse reactions or quality problems they experienced using these devices to MedWatch: The FDA Safety Information and Adverse Event Reporting Program.


