Hair plays an important role in identity, self-perception, and psychosocial functioning. Hair loss can be a devastating experience that decreases self-esteem and feelings of personal attractiveness while also leading to depression and anxiety.1,2 Although increasingly popular, surgical hair restoration, including hair transplantation, is costly and carries considerable risk.
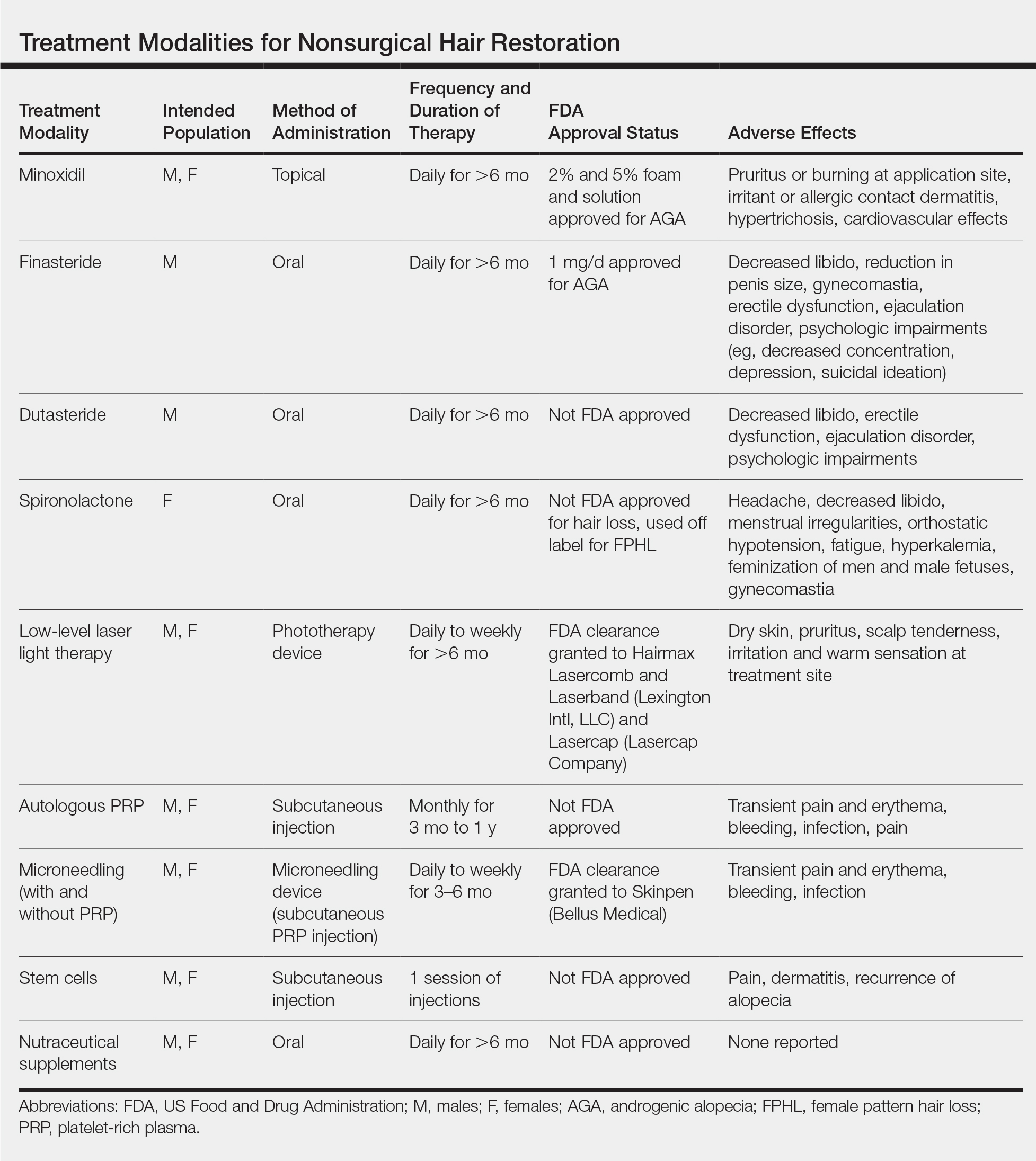
Results of nonsurgical hair restoration are not immediate and may not be as dramatic; however, they do not carry the risks or recovery associated with surgical options. Treatments such as sex steroid hormone and biologic response modifiers have been used to inhibit hair miniaturization and stabilize hair loss in cases of androgenic alopecia (AGA).3 Currently, minoxidil and finasteride are the only US Food and Drug Administration (FDA)–approved medications for the treatment of hair loss; however, other nonsurgical treatment options have gained popularity, including dutasteride, spironolactone, low-level laser therapy (LLLT), platelet-rich plasma (PRP), microneedling, stem cells, and nutraceutical supplements. We provide an overview of these treatment options to help dermatologists select appropriate therapies for the treatment of alopecia (Table).
Minoxidil
Minoxidil has been known to improve hair growth for more than 40 years. Oral minoxidil was first introduced for hypertension in the 1970s with a common adverse effect of hypertrichosis; the 2% solution was marketed for AGA shortly thereafter in 1986.4 Minoxidil is a biologic response modifier that is thought to promote hair growth through vasodilation and stimulation of hair follicles into the growth phase.5 In animal studies, topical minoxidil has been shown to shorten telogen, prolong anagen, and increase hair follicle size.6,7 More recently, topical minoxidil was shown to have anti-inflammatory effects by downregulating IL-1, which may confer an additional role in combatting alopecia.8
Minoxidil is FDA approved for treatment of AGA in men and women and often is used as first-line therapy.9 In 3 separate meta-analyses of topical minoxidil, it was shown to be more effective than placebo for treating AGA in men and women, with a notable increase in target area hair growth.10 A study of 777 male patients treated with topical minoxidil 2% found that 45% subjectively experienced new hair growth.11 However, results may vary, and research indicates that higher concentrations are more effective. In a randomized, double-blind, placebo-controlled trial of 381 women with female pattern hair loss (FPHL), minoxidil solution 2% was found to be superior to placebo after 48 weeks, with average changes in nonvellus hair counts of 20.7/cm2 in the minoxidil group vs 9.4/cm2 in the placebo group.12 In a separate meta-analysis, minoxidil solution 5% demonstrated superiority to both the 2% formulation and placebo with a mean change in nonvellus hair counts of 26.0/cm2.13
Minoxidil also has demonstrated promising benefits in preventing chemotherapy-induced alopecia. Although oncologists most often use the scalp cooling method to prevent hair loss by decreasing perfusion and uptake of cytotoxic agents, cost may be prohibitive, as it is often not reimbursable by insurance companies.14,15 On the other hand, minoxidil is easily procured over-the-counter and has been successfully used to decrease the duration of alopecia caused by chemotherapeutic agents such as fluorouracil, doxorubicin, and cyclophosphamide, as well as endocrine therapies used to treat breast cancer in women.16-18 Minoxidil also has been used off label to treat other forms of alopecia, including alopecia areata, telogen effluvium, eyebrow hypotrichosis, and monilethrix; however, there is inconclusive evidence for its efficacy.5,13,19
Compared to other nonsurgical treatments for hair loss, a meta-analysis found that minoxidil was associated with the highest rate of adverse effects (AEs).16,17 Potential side effects include pruritus or burning at the application site; irritant or allergic contact dermatitis; hypertrichosis; and cardiovascular effects, which may be due to the vasodilatory mechanism of action of minoxidil.20 One randomized double-blind study found that while topical minoxidil did not affect blood pressure, it increased heart rate by 3 to 5 beats per minute, caused considerable increases in left ventricular end-diastolic volume, an increase in cardiac output (by 0.751 min-1), and an increase in left ventricular mass (by 5 g m-2). The authors concluded that short-term use is safe in healthy individuals, but providers should ask about history of coronary artery disease to avoid potential cardiac side effects.21
Patients also should be advised that at least 6 months of minoxidil therapy may be necessary.11 Furthermore, measurable hair changes may disappear within 3 months if the patient chooses to discontinue treatment.22 Finally, providers must consider patient perception of improvement and hair growth while on this medication. In one study, although investigator assessments of hair growth and hair count were increased with the use of minoxidil solution 5% compared to placebo, differences in patient assessment of hair growth were not significant at 48 weeks.22 Therefore, dermatologists should address patient expectations and consider additional treatments if necessary.