Participant Self-assessment
Participants assessed their acne lesions using an 11-point rating scale (–5=100% worsening; –4=76%–99% worsening; –3=51%–75% worsening; –2=26%–50% worsening; –1=1%–25% worsening; 0=no improvement; 1=1%–25% improvement; 2=26%–50% improvement; 3=51%–75% improvement; 4=76%–99% improvement; 5=100% acne clear) to compare their acne at each treatment visit and week 10 follow-up with a baseline photograph.
Physician Assessment
The blinded evaluator assessed acne lesions on the face using the same 11-point rating scale that was used for participant self-assessment. For each participant, assessments were made at each treatment visit and week 10 follow-up by comparing baseline photographs.
Safety Evaluation
The WBPRS score, a standardized 6-point scale (0=no pain; 1=hurts a little bit; 2=hurts a little bit more; 3=hurts even more; 4=hurts whole lot; 5=hurt worst),6 was used to evaluate pain toleration during PBBL treatments and was recorded along with adverse events throughout the study.
Statistical Analysis
Based on data from 2 prior studies,3,7 we expected that the favorable clinical outcome of adapalene gel 0.3% and PBBL therapy would be 23% and 78%, respectively. If the adjunctive therapy with PBBL was beneficial, the favorable outcome would be higher than 78%. To be able to detect this difference, the sample size of 11 patients was needed when 5% type I error and 20% type II error were accepted.
Categorical variables were expressed as percentages, while continuous variables were expressed in terms of median (range). The clinical outcomes between both treatment groups were compared using the Wilcoxon signed rank test. A 2-tailed P value of ≤.05 was considered statistically significant. All statistical calculations were performed using STATA software version 10.0.
RESULTS
Baseline Characteristics
Four male and 7 female patients aged 18 to 35 years (median, 23 years) with mild to moderate acne were enrolled in the study. Of the 11 participants, 7 were white, 2 were black, 1 was Asian, and 1 was Latin American. Baseline characteristics of both sides of the face were comparable in all participants (Table 1). Eight participants (73%) completed the study. Two black participants withdrew from the study due to hyperpigmentation following PBBL treatment; 1 participant did not return for follow-up at week 10, as she was out of the country.
Lesion Counts
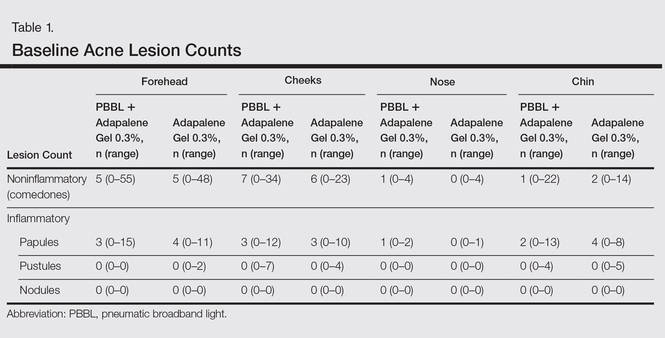
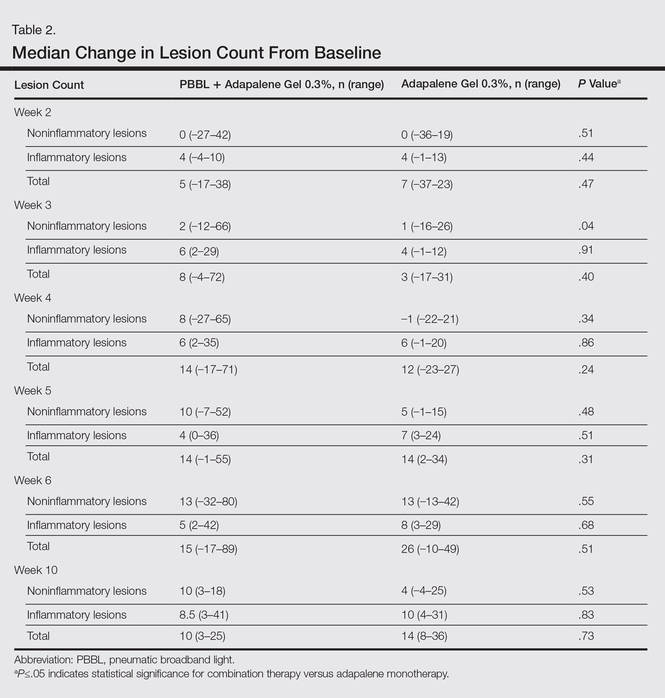
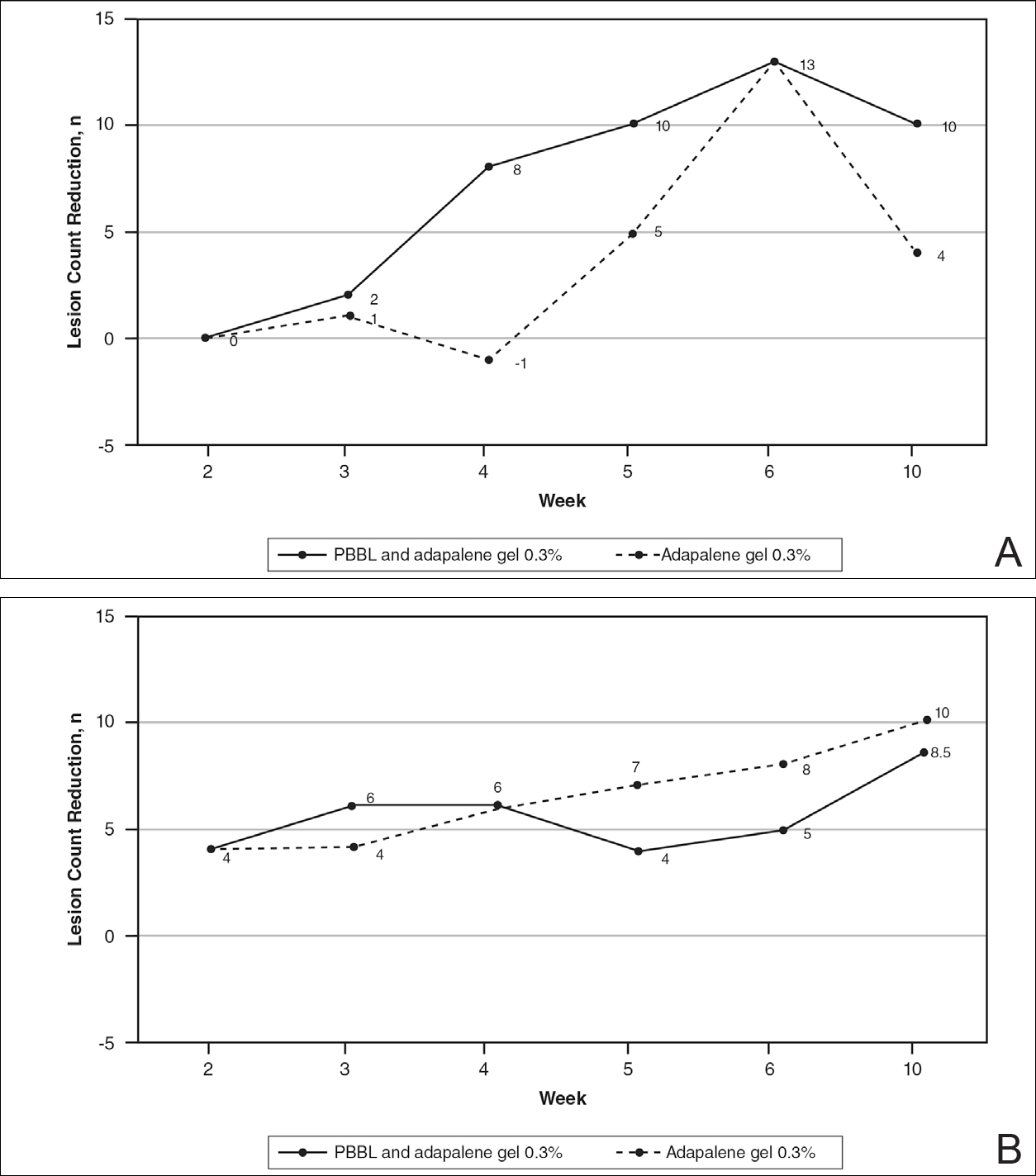
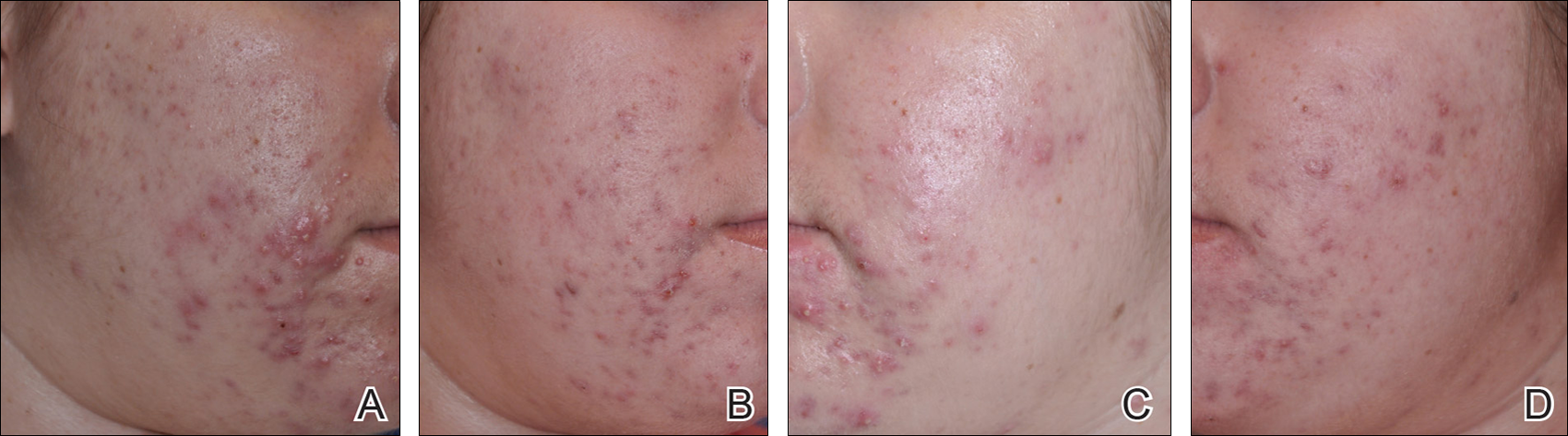
At week 3, reduction in noninflammatory lesions was significantly greater on the side receiving the combination therapy compared to the monotherapy side (P=.04)(Table 2). However, there was no significant difference between the combination therapy and the adapalene monotherapy sides in the reduction of noninflammatory and inflammatory lesions at week 4 (Figure 1). There was a remarkable improvement of the combination therapy and adapalene monotherapy sides in acne lesions, but there was no significant difference between the combination therapy and the adapalene monotherapy sides (Figure 2).
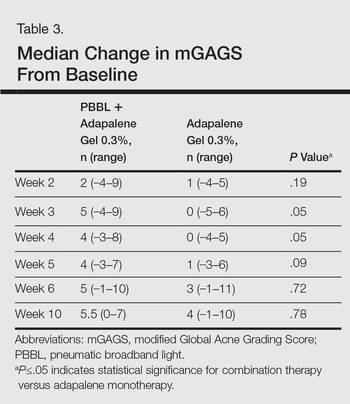
Modified Global Acne Grading Score
At weeks 3 and 4, the improvement of mGAGS was significantly greater on the side treated with the combination therapy (P=.05). However, this significant difference was not sustained (Table 3).
Participant Self-assessment and Physician Assessment
The rate of acne improvement according to participant self-assessment was slightly higher on the side receiving the combination therapy compared to the monotherapy side at week 2 (26%–50% vs 1%–25%) and week 6 (76%–99% vs 51%–75%). However, there was no statistically significant difference. For the physician assessment, there was no significant difference between the monotherapy and combination therapy sides.
Safety
The median WBPRS score was 1 (hurts a little bit) throughout all PBBL treatment visits. The maximum score was highest at week 1 (4=hurts whole lot) and subsequently decreased to 2 (hurts a little bit more) at week 6.
After the PBBL treatment, all participants experienced transient erythema in the treatment area. All participants noted their skin had become drier than usual from adapalene, except 1 participant (11%) who reported very dry skin on areas where adapalene gel 0.3% had been applied. However, the dryness was tolerable and relief was reported following application of a moisturizer. No participants withdrew from the study due to skin dryness.
Both black participants experienced hyperpigmentation caused by PBBL (1 on the treatment sites, the other on the test spot) and withdrew from the study. The hyperpigmentation resolved over time following application of a topical bleaching cream. One patient experienced purpura following PBBL treatment at week 4, which was associated with an increase in PBBL power. No other side effects (eg, scaling, stinging, burning, vesicle formation, blistering, crusting, scarring) were observed.